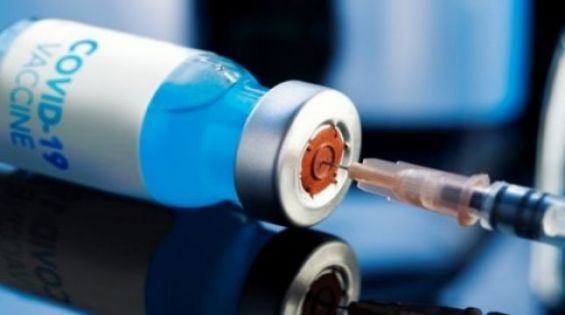
In a few weeks, Morocco will launch a massive Covid-19 vaccination campaign as several laboratories around the world are racing to provide a vaccine to stem the raging pandemic. Researchers are testing 52 vaccines in clinical trials on humans. 87 other preclinical vaccines are under under active investigation in animals, New York Times reported.
«The first vaccine safety trials in humans started in March, and now 10 have reached the final stages of testing», the newspaper added, giving details on Covid-19 vaccines currently tested around the world. And while some of these trials may fail, the good news is that a few vaccines may succeed in boosting the immune system, allowing the human body to produce antibodies against the virus.
4 for China, two for Russia and two for the United States
At least six vaccines were approved for «early or limited use», without waiting for the results of Phase 3 trials. In addition to the four Chinese vaccines (CanSino Biologics, Wuhan Institute of Biological Products, Sinopharm and Sinovac Biotech), two Russian vaccines, including the one developed by the Gamaleya Research Institute (Russian Ministry of Health) are part of this category.
«On October 14, Vladimir Putin announced that Russia has granted regulatory approval to EpiVacCorona, making it the second vaccine to receive that designation after the Gamelaya Institute’s Sputnik V vaccine», recalls New York Times.
But there are other vaccines that have started phase 3 efficacy tests. They include a vaccine from American Moderna, a partner of the National Institutes of Health in the United States. Based on messenger RNA (mRNA) to produce viral proteins in the body, this vaccine progressed into phase 3 trials, which began on July 27.
The list also includes the vaccine from Pfizer and the German laboratory BioNTech, which announced earlier this week that their vaccine is more than 90 % effective. There is also the Johnson & Johnson’s vaccine, which is supported by the United States government. Phase 3 tests were paused briefly in October to investigate an adverse reaction in a volunteer. This company expects to see results by the end of the year.
AstraZeneca, Novavax and other laboratories
British-Swedish multinational pharmaceutical and biopharmaceutical company AstraZeneca and the University of Oxford are also developing a vaccine based on a chimpanzee adenovirus called ChAdOx1. On September 6, AstraZeneca also halted global vaccine trials to investigate a volunteer, who experienced side effects. But a week later, the tests were resumed.
Novavax, based in the US state of Maryland, is also working on a vaccine. After obtaining promising results from preliminary tests on monkeys and humans, and the launch of phase 2 in South Africa in August, Novavax launched a phase 3 trial recruiting up to 15,000 volunteers in the UK. A larger Phase 3 trial is under development and will be launched in the United States by the end of November.
Two other vaccines come from India and Australia. In collaboration with the Indian Council for Medical Research and the National Institute of Virology, the Indian company Bharat Biotech has designed a vaccine called Covaxin based on an inactivated form of the coronavirus. On October 23, the company announced that it is entering a phase 3 trial. Meanwhile, the Murdoch Children's Research Institute in Australia is also conducting a phase 3 trial called BRACE to see if the vaccine partially protects against the coronavirus.
It is worth mentioning that Morocco, in addition to its partnerships with Sinopharm and AstraZeneca, is in negotiations with other suppliers to acquire doses of a potential vaccine.





 chargement...
chargement...