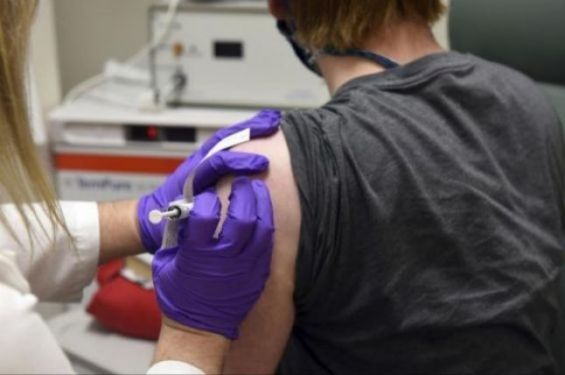
The Medicines and Healthcare products Regulatory Agency (MHRA), an executive agency of the Department of Health and Social Care in the United Kingdom, has granted emergency authorization to the vaccine made by Pfizer and BioNTech.
According to The Economist, the vaccine is the first fully-tested covid-19 one that has been authorized for use in Britain, after trials have shown that it has an efficacy of 95%.
Ugur Sahin, head of BioNTech, said the move would reduce hospitalization for people in the high-risk population. Businesses anticipate further decisions of this type in the days and weeks to come and say this marks a historic milestone in the fight against covid-19.
The Economist reports that Britain has ordered 40 million doses of Pfizer and BioNTech's mRNA vaccine, known as BNT162b2.
The regulator's decision will be followed by the arrival of vaccines. «These are expected to come from a manufacturing site in Puurs, Belgium, and will be delivered in stages this year and throughout 2021», the same source added.
The same source also reports that the authorities have invited hospitals to be ready for a vaccine distribution as of December 7. «In the meantime, the UK Joint Committee on Vaccination and Immunization will have to make a final decision on priority groups for vaccination, such as health workers and people at high risk of covid-19», it concluded.





 chargement...
chargement...