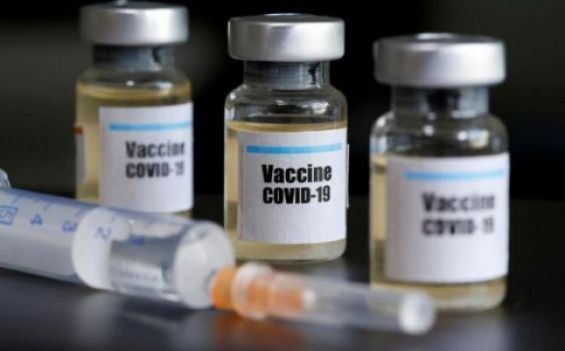
Pfizer's Covid-19 vaccine took a new step on Thursday when a group of American experts formally recommended that the Food and Drug Administration (FDA) authorize the vaccine.
The agency will likely do so in a few days, prioritizing healthcare workers and residents of nursing homes to begin receiving the vaccine next week, the New York Times reported.
The FDA's Vaccine Advisory Group, made up of independent scientific experts, infectious disease physicians and statisticians, had 17 of its members vote in favor of emergency clearance for 16-year-olds, 4 opposed the authorization and one member abstained.
With this formal blessing, the United States can finally begin to slow the spread of Covid-19 as infections and deaths increase.
«The FDA is expected to grant emergency use authorization on Saturday», according to sources familiar with the agency's planning. But they warn, however, against «last minute legal or bureaucratic requirements that could push the announcement to Sunday or later».
Great Britain was the first country to authorize the vaccine from Pfizer and BioNTech, following a decision by its Medicines and Health Products Regulatory Agency (MHRA). However, the British health authorities on Wednesday advised against inoculating the vaccine to people who have had «significant allergic reactions» in the past, after two people reacted adversely to the first injections.





 chargement...
chargement...