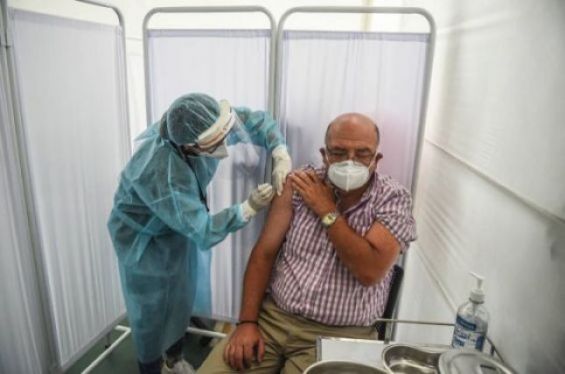
The Peruvian government announced on Wednesday that China's Sinopharm could resume a trial for its coronavirus vaccine.
According to Reuters, the country's authorities said they had «clarified» the problem that affected one of the 12,000 volunteers who received the vaccine as part of the trials.
The authorities in Lima announced, Friday, December 11, to have «temporarily suspended» the clinical trials of the Sinopharm vaccine after the detection of neurological problems in one of the volunteers.
Sinopharm was close to concluding the first part of its testing, with plans to inoculate a second dose of the vaccine over the next three weeks, the researchers said.
«We have had several meetings with Sinopharm and... the suspension has been lifted today (Wednesday)», Health Minister Pilar Mazzetti said.
For the record, Morocco is preparing to launch its vaccination campaign against Covid-19, in particular with the vaccine developed by the Chinese Sinopharm.




 chargement...
chargement...