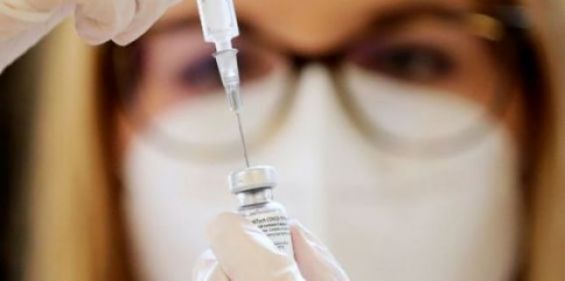
The drug registration commission in Morocco launched this Thursday the approval procedure for anti-coronavirus vaccines, developed by AstraZeneca and Sinopharm, selected by the Kingdom for the launch of its vaccination campaign.
An authorized source quoted by Medias24 reports that the final market launching authorization would be granted within a week or so.
Morocco was awaiting the authorization of these vaccines in their countries of origin, China and the United Kingdom, before launching a procedure at the national level. The vaccine developed by the University of Oxford for British-Swedish group AstraZeneca was authorized yesterday by the UK’s health authorities.
For its part, China has authorized Sinopharm's vaccine, developed by its state-owned CNBG subsidiary in Beijing, but has not yet authorized the Wuhan-produced vaccine.




 chargement...
chargement...