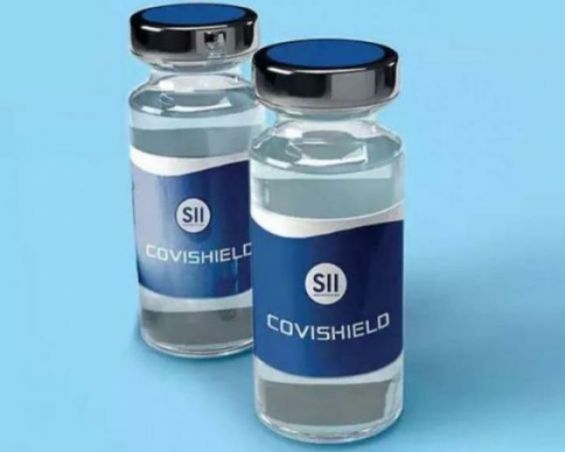
Morocco granted, Tuesday, a temporary authorization for the use of the AstraZeneca vaccine, developed in collaboration with the University of Oxford.
According the authorization signed by Health Minister Khalid Ait Taleb, the authorized vaccine is called «COVISHIELD».
The authorization is, in fact, granted to the Serum Institute Of India Pvt Ltd, the world's largest vaccine manufacturer licensed by AstraZeneca.
The «vaccination schedule against the COVID-19 vaccine consists of two separate doses of 0.5 ml each», while the second dose should be administered between 4 and 12 weeks after the first one.
The same source also notes that this vaccine is intended for intramuscular (IM) injection only, preferably in the deltoid muscle and that it must be stored in the refrigerator (between 2 ° C and 8 ° C).
The document indicates that this authorization is valid for a period of 12 months, starting from the date of its signature.





 chargement...
chargement...