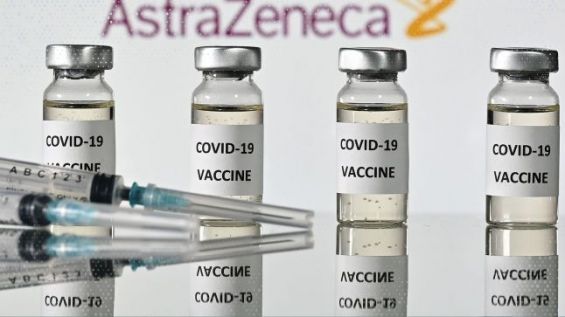
Indian biotechnology and pharmaceuticals company Serum Institute of India (SII) is expected to receive World Health Organization (WHO) emergency use authorization soon for the Oxford University/ AstraZeneca coronavirus vaccine.
The firm will be producing said vaccine for mid and low income countries, including Morocco, Reuters reported, quoting SII’s chief executive.
«The emergency use licensure from the WHO should be available and coming through in the next week or two, hopefully, because we have submitted everything», Adar Poonawalla said of the AstraZeneca/Oxford vaccine during a Reuters Next conference.
Poonawalla said that SII has been approached by Morocco for the AstraZeneca vaccine. It is not clear when the delivery would reach Morocco.
For the record, the head of Morocco’s National Doctors group told Yabiladi earlier this week that the vaccination campaign in the Kingdom will likely start by next week.
It is worth mentioning that Morocco authorized the use of the AstraZeneca vaccine, developed in collaboration with the Oxford University. The Moroccan government announced it had ordered 65 million doses of COVID-19 vaccines from China's Sinopharm and Britain's AstraZeneca.





 chargement...
chargement...