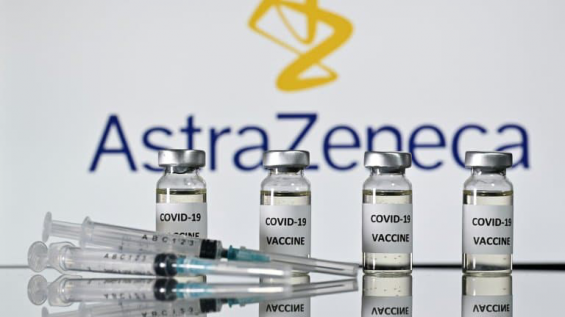
A major paving stone in the AstraZeneca pond. According to sources within the German government majority, cited by Bild and Handelsblatt, the Swedish-British anti-Covid-19 vaccine would be ineffective (less than 10%) for people over 65. A revelation that calls into question the place of the vaccine developed by the University of Oxford in the vaccine strategy of the countries that have authorized it.
The pharmaceutical company refuted the assertions of the two German media, calling the allegations «totally incorrect». The vaccine producer cites as evidence the study published in scientific journal The Lancet and the authorization, for all ages, granted by the British health authorities.
The German government does not expect an authorization for the vaccine from the European Medicines Agency for the population over the age of 65. The regulatory green light from the European Union is expected for Friday, January 29.
A standoff has been playing out for a few days between Brussels and AstraZeneca which announced that deliveries would be lower than expected in the first quarter, due to a «production yield drop» in one of its European manufacturing sites.
The President of the European Commission, Ursula von der Leyen «stated very clearly that she expects AstraZeneca to honor the contracts and conditions provided for in the pre-order agreement», said a Commission spokesperson. «Production problems may appear, but we expect the company to find solutions», she added.
This blow to the reputation of AstraZeneca's vaccine could also be bad news for Morocco, which has just received 2 million doses produced in India by the Serum Institute of India. At the center of its vaccine strategy, which is due to start this week, if the low efficacy for people over 65 is confirmed, the Kingdom would only be able to count on the Sinopharm vaccine for this category of people.





 chargement...
chargement...