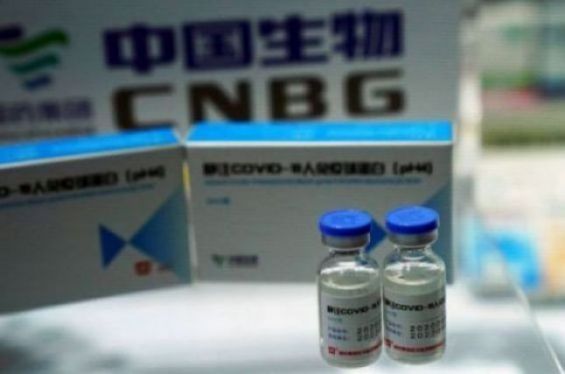
As of January 25, 2021, the schedule of controls for vaccines against the new coronavirus, published by the World Health Organization (WHO), indicates that the UN institution has not yet approved the injectable formula developed by Sinopharm’s laboratories in Wuhan. This grid, accessible on the organization's platforms, details all of the vaccines under review.
These include Covishield, developed by the Serum Institute of India under license from AstraZeneca/University of Oxford and acquired by Morocco for the launch of its national vaccination campaign.
The evaluation is in its final stages and the anticipated approval date is set for mid-February, meaning it has already met most of the efficacy and safety criteria.
The same applies for the version of the Chinese vaccine from Sinopharm developed in Beijing, scheduled to be authorized in March. The study of the file relating to the Wuhan one remains at a standstill, because it has not been yet submitted by the laboratory.
This situation could explain the delay in delivery for Morocco, while the Kingdom participated in the clinical trials of the third phase of experimentation with the vaccine developed in Wuhan. At the national level, the data showed positive indicators as to the effectiveness or the risk of these injections. But this vaccine still hasn't seen the end of the tunnel.





 chargement...
chargement...