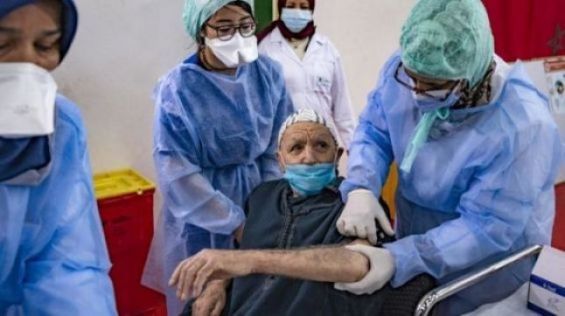
The ad hoc national scientific committee for the development of the vaccine strategy against Sars-Cov-2, met this Thursday in Rabat to give its opinion on the use of the AstraZeneca vaccine in people over 65 years of age. In a press release sent to Yabiladi, the Ministry of Health indicates that Morocco is maintaining the use of this vaccine for this age group, as initially recommended in the national anti-Covid-19 vaccination strategy.
The same source indicates that following questions raised in recent days concerning the efficacy and safety of the AstraZeneca vaccine in the population aged 65 and over, the committee considered that «the anti-Covid vaccine developed by AstraZeneca has obtained the authorization for use, without age limit at 65, by the European Medicines Agency and the health authorities of several countries». In addition, «the phase 3 clinical trial of this vaccine includes a subgroup of people over 65 years of age among whom 687 people received the vaccine, 4 of whom developed Covid-19 and 666 people in the control group 7 of whom have developed Covid-19», the press release recalls.
For the committee, as of today, «the data from this clinical trial does not allow conclusions to be drawn regarding the exclusion of this age group from beneficiaries of this vaccine». The same source explains that «protection is expected for this age group, given that high seroconversion rates have been observed in adults over 65 years after the first dose (97.8%) and the second dose (100%)».
As for the safety of said vaccine, «the published data shows that the reported side effects are generally lighter and less frequent in people over 65 years of age compared to the youngest», the committee concludes.




 chargement...
chargement...