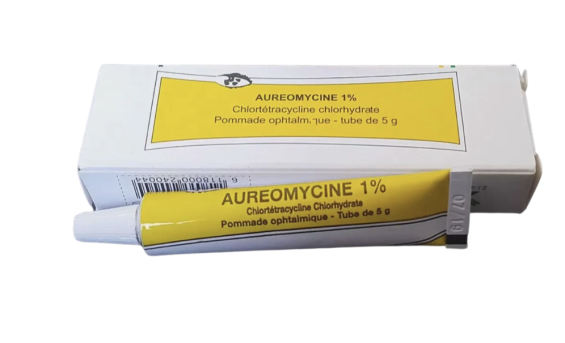
The Moroccan Agency for Medicines and Health Products has ordered the immediate withdrawal of several batches of the ointment «Oriumycin 1%», commonly known as «the yellow ointment», following the detection of a quality defect during laboratory testing.
In an official letter to the Moroccan Pharmaceutical Promotion Company «Promopharm», the agency reported that tests revealed a defect in the consistency or appearance of the ointment, which could pose a potential risk to consumer health. The batches concerned are 22043, 22046, and 1001, all of which must be withdrawn nationwide.
The agency also instructed wholesale distributors, retail pharmacies, and the logistics departments of healthcare institutions to immediately stop marketing the product and to return all available quantities without delay.
Additionally, it requested a detailed report on the progress of the withdrawal process, including a comparison between the quantities sold and those recovered, along with official documentation proving the safe destruction of the defective products.
Notably, Oriumycin is one of the most widely used topical antibiotics in Morocco for treating minor eye infections.





 chargement...
chargement...